Disclaimer: This page is intended for healthcare professionals only.
About Weliva® Cimidona®
Weliva® Cimidona® is an over-the-counter product, available without a prescription. This hormone-free solution for adult women at any stage of menopause contains a naturally sourced active ingredient to help reduce the frequency and severity of menopausal symptoms.1
Other key points about Weliva® Cimidona® that can help guide your recommendation:
Indicated for adult women in any stage of menopause1 to help with the effective relief of multiple symptoms related to menopause1
Contains Ze 450, an exclusive extract of Actaea racemosa
Helps to reduce the frequency and severity of menopausal symptoms, including hot flashes, night sweats, fatigue, irritability, nervousness, sleeplessness, mild joint pain, concentration weakness, palpitations, and headache1,2
Available as 13-mg tablets1
Recommended dosage is 1 tablet once a day1
Patients can expect to feel the effects of Weliva® Cimidona® after a minimum of 6 weeks of use.1
*Natural Product Number (NPN) 80125992.1
Understanding Ze 450
Actaea racemosa (also known as black cohosh)1 is a perennial medicinal plant that has traditionally been used to treat various conditions, including menopause-related symptoms.2
While the mechanism of action of Actaea racemosa has yet to be fully elucidated, evidence indicates that the Ze 450 extract acts through non-estrogenic, neuro-hormonal pathways. Experimental studies show partial agonist activity at serotonin (5-HT1A, 5-HT1D, 5-HT7) and µ opiate receptors, which are involved in hypothalamic thermoregulation. By modulating these receptors, Ze 450 may stabilize the temperature-control centre of the brain and thereby reduce the frequency and intensity of hot flushes.2,7
Since In vitro studies have shown that Ze 450 does not mediate any estrogenic effects, it could be considered a non-hormonal alternative to menopausal hormone therapy (MHT).4,5

Weliva® Cimidona® Ze 450 has been studied in multiple clinical studies
The efficacy and safety of Ze 450 has been clinically demonstrated in one randomized, placebo-controlled trial, one open-label prospective trial and one retrospective observational study.
The research at a glance:
| STUDY | DESIGN | TREATMENT‡ | MAIN OUTCOME ANALYZED |
|---|---|---|---|
| Schellenberg 20122 | 12-week, multicentre, randomized, double-blind, placebo-controlled, parallel-group trial*
n=180 |
Cimidona® Ze 450 13 mg Placebo | Primary Endpoint Difference in menopausal symptom severity as assessed by a modified total Kupperman Menopausal Index (KMI) score from baseline to Week 12 |
| Drewe 20138 | 12-week, multicentre, open-label, prospective, observational study with an additional 6-month extension†
n=422 |
Cimidona® Ze 450 13 mg | Primary Endpoint Difference in menopausal symptom severity as assessed by a modified total (KMI) score from baseline to Week 12 |
| Friederichsen 20203 | Monocentric, retrospective, observational cohort study†
n=174 |
Cimidona® Ze 450 13 mg or Menopausal hormone therapy (MHT) | Metabolic serum parameters, body weight, and menopausal symptoms as assessed by the Menopause-Rating-Scale (MRS-II) |
*Schellenberg (2012): Treatment arms were Ze 450 6.5 mg (n=57), and Ze 450 13 mg (n=55), and placebo (n=54). The primary comparison was between the 13 mg group and the placebo group (totaling 109 participants). Primary outcome was the change in menopausal symptoms by the Kupperman Menopausal Index.2
†Drewe (2013): Ze 450 at doses of 1x 6.5 mg or 2x 13 mg dry extract were also used. In Friederichsen study (2019), Ze 450 at doses of 1x 6.5 mg was also used.3,8
†‡Only Weliva® Cimidona® 13 mg is approved for use in Canada.1
Schellenberg (2012) study2*
Snapshot of the trial:
Schellenberg studied 180 women >40 years with menopausal complaints over 12 weeks.2 This 12-week, multicentre, randomized, double-blind, placebo-controlled, parallel-group trial (n=109) evaluated the efficacy of extract Ze 450 from Actaea racemosa (Ze 450, 13 mg, n=55†‡) versus placebo (n=54). The primary endpoint was the difference in menopausal symptom severity, measured using a modified total (KMI) score from baseline to Week 12.2
Ze 450 13 mg significantly reduced the symptoms of menopause§
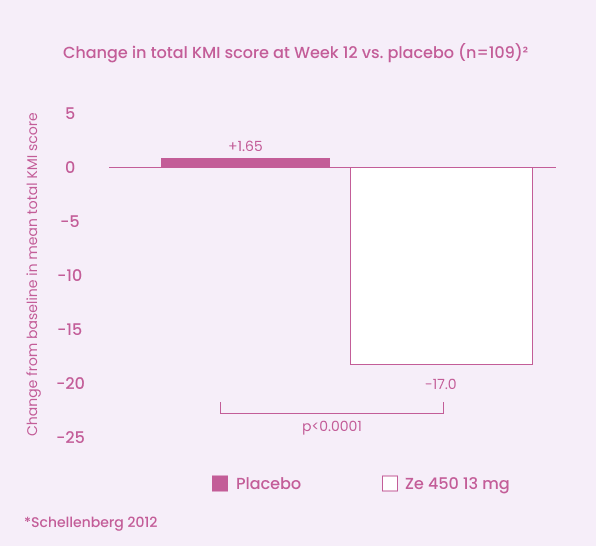
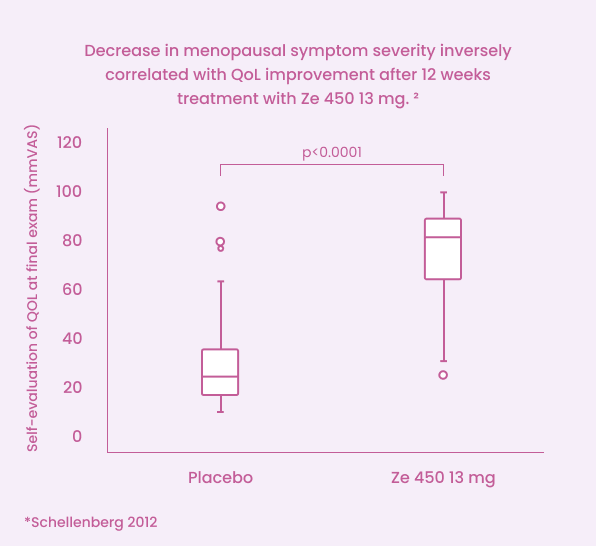
Ze 450 was superior to placebo in decreasing the overall menopausal symptom severity (vasomotor, psychological, and somatic)2
Key points:
Significant reduction in severity of menopausal symptoms (vasomotor, psychological and somatic) compared to placebo2
Significant improvement in self-assessed quality of life (QoL)2¶
Ze 450 shown to be an effective and well-tolerated nonhormonal treatment option for menopausal symptom relief2
*Randomized, double-blind, placebo-controlled study - compared to placebo (p < 0.0001) Patients took the Ze 450 extract (13 mg) or placebo daily for a period of 12 weeks. The primary outcome was the difference in menopausal symptoms experienced (vasomotor, psychological and somatic) observed between Week 12 and the established baseline.2
†Schellenberg (2012): Ze 450 at a dose of 6.5 mg dry extract was also a treatment arm (n=57).2
‡Only Weliva® Cimidona® 13 mg is approved for use in Canada.1
§Data are shown for those who received Ze 450 13 mg only. Only Weliva® Cimidona® 13 mg is approved for use in Canada.1
¶ Assessed using a 100 mm visual analog scale.2
Drewe (2013) study8*
Snapshot of the study:
Drewe observed 442 women with menopausal complaints over 12 weeks.8 This 12-week, multicentre, open-label, prospective, observational study with an additional 6-month extension (n=228) evaluated extract Ze 450 from Actaea racemosa (Ze 450, 13 mg†‡ ). The primary endpoint was the change in menopausal symptom severity, measured using a modified total (KMI) score from baseline to Week 12.8
Long-term menopausal symptom relief with Cimidona® Ze 450.
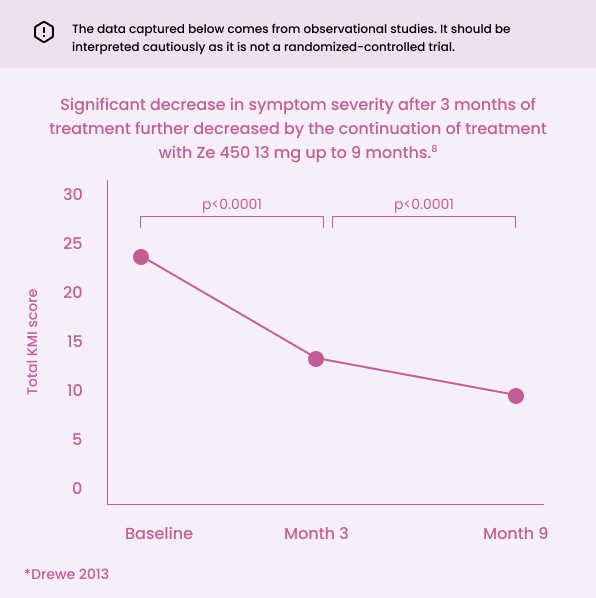
Key points:
Significant reduction in the severity of menopausal symptoms after 3 months of treatment8
Continued treatment improved menopausal symptoms with significant relief at 9 months8
Ze 450 was very well-tolerated8
*Observational study - (p < 0.001) Patients took 1 Cimidona® 13 mg tablet daily for a period of 3 months. In the 6 months extension that followed, some patients switched to 6.5 mg dosage (n=102), while others continued on the 13 mg dose (n=228). The outcomes measured the total menopausal severity scores and its sub-items at 3 months and at 9 months compared to the baseline.8
†Drewe (2013): Ze 450 at doses of 1x 6.5 mg (n=27 in phase 1, n=131 in phase 2) or 2x 13 mg (n=23 in phase 1, n=12 in phase 2) dry extract were also used.8
‡Only Weliva® Cimidona® 13 mg is approved for use in Canada.1
Friederichsen (2019) study3*
Snapshot of the study:
Friederichsen retrospectively studied 174 women >40 years with menopausal complaints.3 This monocentric, observational cohort study compared Ze 450 from Actaea racemosa (Ze 450, 1x13 mg (n=25) or 1x6.5 mg (n=7)†‡ ) with menopausal hormone therapy (MHT) (n=142). Outcomes included metabolic serum parameters, body weight, and menopausal symptoms assessed using the Menopause Rating Scale (MRS-II).3
Ze 450 13 mg reduced menopausal symptom severity with no evidence of difference vs. MHT.
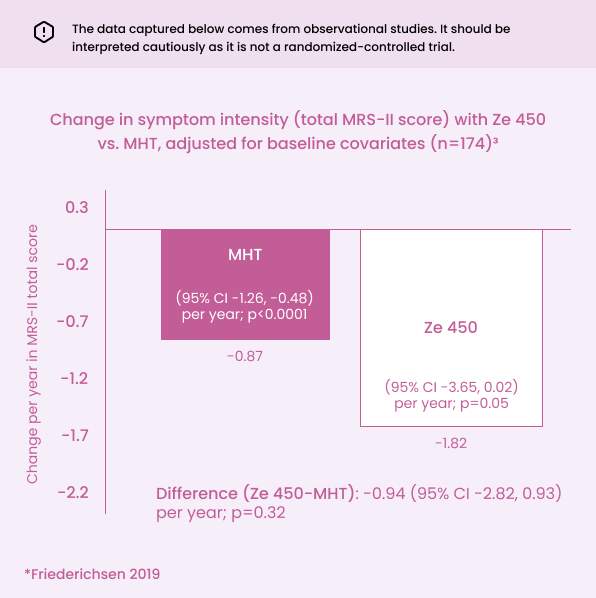
Both Ze 450 and MHT reduced menopausal symptom severity with no evidence of between-group difference.3
Key points:
Significant improvements in menopausal symptoms in both groups3
No changes in body weight were observed during the study period in either group3
*Monocentric retrospective cohort study: Women with menopausal symptoms who had their first consultation between 2009 and 2016 were screened. Eligible participants were those treated with either MHT or extract Ze 450 from Actaea racemosa (Cimidona®) and who had at least one follow-up consultation during the 12-month observation period. Treatment groups included Ze 450 at 6.5 mg (n=7) or 13 mg (n=25), and MHT (n=142). Outcomes were assessed retrospectively, focusing on comparisons between Ze 450 (both doses combined) and MHT with respect to metabolic parameters and body weight. Primary outcome measures included metabolic serum parameters (lipids, glucose, insulin, and HOMA-IR), body weight, and menopausal symptoms assessed by the Menopause Rating Scale (MRS-II). Statistical analysis employed uni- and multivariable linear mixed-effects regression models assuming a linear effect of time.3
†Only Weliva® Cimidona® 13 mg is approved for use in Canada. In this study, for the 13 mg dose, Cimifemin® forte and Climavita® Forte were used. These are European brand names that are equivalent to Cimidona® in Canada.3
Weliva® Cimidona® Ze 450 13 mg was generally well tolerated2,8
Clinical trials support a positive safety profile of Weliva® Cimidona® for up to 9 months of treatment.2,8 Treatment-related adverse events were uncommon and non-serious. The most common complaints were gastrointestinal in nature (e.g., nausea and stomach pain).2,8
Advising your patients on taking Weliva® Cimidona®
Counsel patients to take 1 tablet once a day,1 and to use for a minimum of 6 weeks to see beneficial effects.1
Advise your patients to consult you and/or their pharmacist if their symptoms persist or worsen, or if they have a liver disorder or develop symptoms of liver trouble.
Do not recommend for your patients who are pregnant or breastfeeding.1
References:
- Health Canada. Weliva® Cimidona® Product Licence. Issued, August 4, 2023.
- Schellenberg R, Saller R, Hess L, et al. Dose-Dependent Effects of the Cimicifuga racemosa Extract Ze 450 in the Treatment of Climacteric Complaints: A Randomized, Placebo-Controlled Study. Evid Based Complement Alternat Med. 2012;2012:260301. doi:10.1155/2012/260301
- Friederichsen L, Nebel S, Zahner C, Bütikofer L, Stute P. Effect of Cimicifuga racemosa on metabolic parameters in women with menopausal symptoms: a retrospective observational study (CIMBOLIC). Arch Gynecol Obstet. 2020;301(2):517–523. doi:10.1007/s00404-019-05366-8
- Garita-Hernandez M, Calzado MA, Caballero FJ, et al. The growth inhibitory activity of the Cimicifuga racemosa extract Ze 450 is mediated through estrogen- and progesterone-receptor–independent pathways. Planta Med. 2006;72(4):317–323. doi:10.1055/s-2005-916233
- Wuttke W, Seidlová-Wuttke D. Black cohosh (Cimicifuga racemosa) is a non-estrogenic alternative to hormone replacement therapy. Clinical Phytoscience. 2015;1(1):12. doi:10.1186/s40816-015-0013-0
- Drewe J, Bucher KA, Zahner C. A systematic review of non-hormonal treatments of vasomotor symptoms in climacteric and cancer patients. Springerplus. 2015;4:65. Published 2015 Feb 10. doi:10.1186/s40064-015-0808-y
- Rhyu MR, Lu J, Webster DE, Fabricant DS, Farnsworth NR, Wang ZJ. Black cohosh (Actaea racemosa, Cimicifuga racemosa) behaves as a mixed competitive ligand and partial agonist at the human mu opiate receptor. J Agric Food Chem. 2006;54(26):9852–9857. doi:10.1021/jf062808u
- Drewe J, Zimmermann C, Zahner C. The effect of a Cimicifuga racemosa extract Ze 450 in the treatment of climacteric complaints—an observational study. Phytomedicine. 2013;20(8–9):659–666. doi:10.1016/j.phymed.2013.02.012
Disclaimers:
- Always direct the patient to read the label. For more information, please consult Warnings, Cautions, and Directions of Use available https://health-products.canada.ca/lnhpd-bdpsnh/info?licence=80034529 for information to assist in benefit-risk assessment. The terms of Market Authorization are also available upon request by calling us at 1- 888-550-6060.


